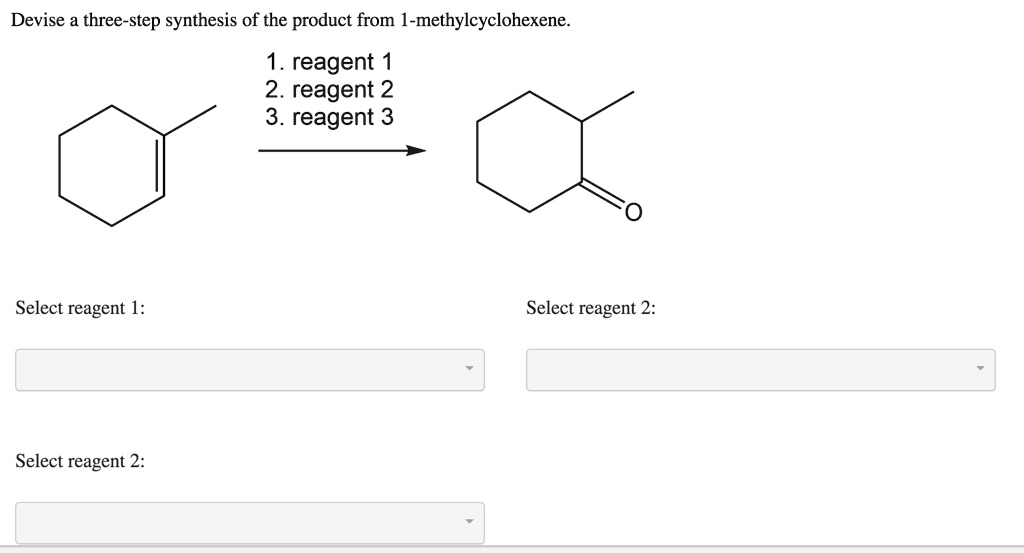
Science Chemistry Chemistry questions and answers Devise a three-step synthesis of the product from 1-methylcyclohexene. 1. reagent 1 2. reagent 2 3. reagent 3 a This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
SOLVED: Devise a 2-step synthesis of the product from the starting material. reagent 2. reagent 2 CH3 “OH “H CH3 Select reagent 1: Select reagent 2: CH3
Step 1: Mapping starting materials onto the product Step 2: Identify key bonds to be made Step 3: Consider how to make the key bond (s) Step 4. Adjust functional groups Step 5: Write out the whole synthesis and check it carefully Let’s assume you want to make a particular product.

Source Image: pubs.acs.org
Download Image
Step 1 Alkenes are those hydrocarbons that contain at least one π π -bond. Since sigma bonds are stronger than… View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text: Devise a three-step synthesis of the product from 1-methylcyclohexene. 1.

Source Image: homework.study.com
Download Image
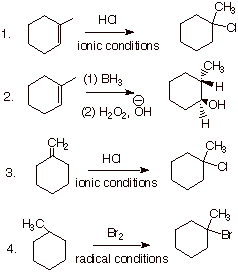
Chemistry of the Secondary Metabolites of Termites | SpringerLink A strong base with heat can be used for the second step to follow an E2 mechanism and form 1-methylcyclohexene. The aldehyde group on the final product indicates gentle oxidative cleavage by any of several reaction pathways. These reactions can be combined in to the following multi-step synthesis.
Source Image: organicsynthesisinternational.blogspot.com
Download Image
Devise A Three-Step Synthesis Of The Product From 1-Methylcyclohexene
A strong base with heat can be used for the second step to follow an E2 mechanism and form 1-methylcyclohexene. The aldehyde group on the final product indicates gentle oxidative cleavage by any of several reaction pathways. These reactions can be combined in to the following multi-step synthesis. Question: (a) Draw the products (including stereoisomers) formed when 2-methylhex-2-ene is treated with HBr in the presence of peroxides. (b) Draw the products (including stereoisomers) formed when (S)-2,4-dimethylhex-2-ene is treated with HBr and peroxides under similar conditions.
Organic Synthesis International: 1- Methyl cyclohexene, All about it pictoral
The three-step synthesis of the product from 1-methylcyclohexene is as follows: converted into 1-bromo-1-methylcyclohexane with HBr, use NaNH2 (sodium amide) with the product obtained from step 1 and treat the obtained intermediate from step 2 with D2O (heavy water) Development of a Scalable Synthetic Route to BMS-986251. Part 1: Synthesis of the Cyclohexane Dicarboxylate Fragment | Organic Process Research & Development

Source Image: pubs.acs.org
Download Image
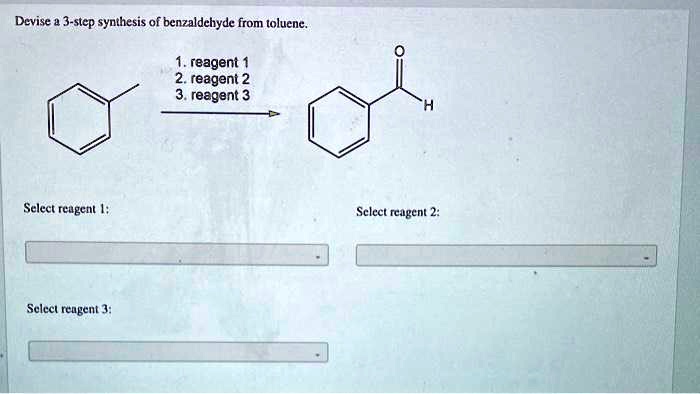
SOLVED: Devise a 3-step synthesis of benzaldehyde from toluene. Reagent 1 Reagent 2 Reagent 3 Select reagent Select reagent 2 Select reagent 3: The three-step synthesis of the product from 1-methylcyclohexene is as follows: converted into 1-bromo-1-methylcyclohexane with HBr, use NaNH2 (sodium amide) with the product obtained from step 1 and treat the obtained intermediate from step 2 with D2O (heavy water)
Source Image: numerade.com
Download Image
SOLVED: Devise a 2-step synthesis of the product from the starting material. reagent 2. reagent 2 CH3 “OH “H CH3 Select reagent 1: Select reagent 2: CH3 Science Chemistry Chemistry questions and answers Devise a three-step synthesis of the product from 1-methylcyclohexene. 1. reagent 1 2. reagent 2 3. reagent 3 a This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: numerade.com
Download Image
Chemistry of the Secondary Metabolites of Termites | SpringerLink Step 1 Alkenes are those hydrocarbons that contain at least one π π -bond. Since sigma bonds are stronger than… View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text: Devise a three-step synthesis of the product from 1-methylcyclohexene. 1.

Source Image: link.springer.com
Download Image
Synthetic Routes, 3 Step synthesis : r/chemhelp Jun 26, 2023To devise a three-step synthesis of the product from 1– methylcyclohexene, you can follow these steps: Step 1: Perform a hydroboration reaction. In this step, you can react 1-methylcyclohexene with borane (BH3) in the presence of a solvent, such as tetrahydrofuran (THF).

Source Image: reddit.com
Download Image
SOLVED: Devise a three- step synthesis of the product from 1- methylcyclohexene reagent 2. reagent 2 3. reagent 3 Select reagent 1: Select reagent 2: Select reagent 2: A strong base with heat can be used for the second step to follow an E2 mechanism and form 1-methylcyclohexene. The aldehyde group on the final product indicates gentle oxidative cleavage by any of several reaction pathways. These reactions can be combined in to the following multi-step synthesis.
Source Image: numerade.com
Download Image
Solved Provide the mechanism for formation of 3 products: | Chegg.com Question: (a) Draw the products (including stereoisomers) formed when 2-methylhex-2-ene is treated with HBr in the presence of peroxides. (b) Draw the products (including stereoisomers) formed when (S)-2,4-dimethylhex-2-ene is treated with HBr and peroxides under similar conditions.

Source Image: chegg.com
Download Image
SOLVED: Devise a 3-step synthesis of benzaldehyde from toluene. Reagent 1 Reagent 2 Reagent 3 Select reagent Select reagent 2 Select reagent 3:
Solved Provide the mechanism for formation of 3 products: | Chegg.com Step 1: Mapping starting materials onto the product Step 2: Identify key bonds to be made Step 3: Consider how to make the key bond (s) Step 4. Adjust functional groups Step 5: Write out the whole synthesis and check it carefully Let’s assume you want to make a particular product.
Chemistry of the Secondary Metabolites of Termites | SpringerLink SOLVED: Devise a three- step synthesis of the product from 1- methylcyclohexene reagent 2. reagent 2 3. reagent 3 Select reagent 1: Select reagent 2: Select reagent 2: Jun 26, 2023To devise a three-step synthesis of the product from 1– methylcyclohexene, you can follow these steps: Step 1: Perform a hydroboration reaction. In this step, you can react 1-methylcyclohexene with borane (BH3) in the presence of a solvent, such as tetrahydrofuran (THF).