The viscosity of a liquid is a measure of its resistance to flow. Water, gasoline, and other liquids that flow freely have a low viscosity. Honey, syrup, motor oil, and other liquids that do not flow freely, like those shown in Figure 5.3.1 5.3. 1, have higher viscosities. We can measure viscosity by measuring the rate at which a metal ball
Four guiding principles for choosing frameworks and indicators to assess research impact | Impact of Social Sciences
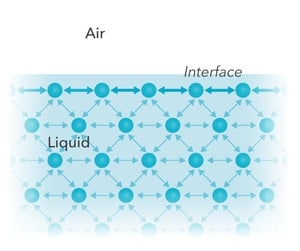
5 days agoSurface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it.The molecules in a drop of water, for example, attract each other weakly. Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules.

Source Image: biolinscientific.com
Download Image
This page titled 6.7: Cohesion and Adhesion in Liquids – Surface Tension and Capillary Action is shared under a CC BY license and was authored, remixed, and/or curated by OpenStax. Attractive forces between molecules of the same type are called cohesive forces. Attractive forces between molecules of different types are called adhesive forces.

Source Image: togwt1980.blogspot.com
Download Image
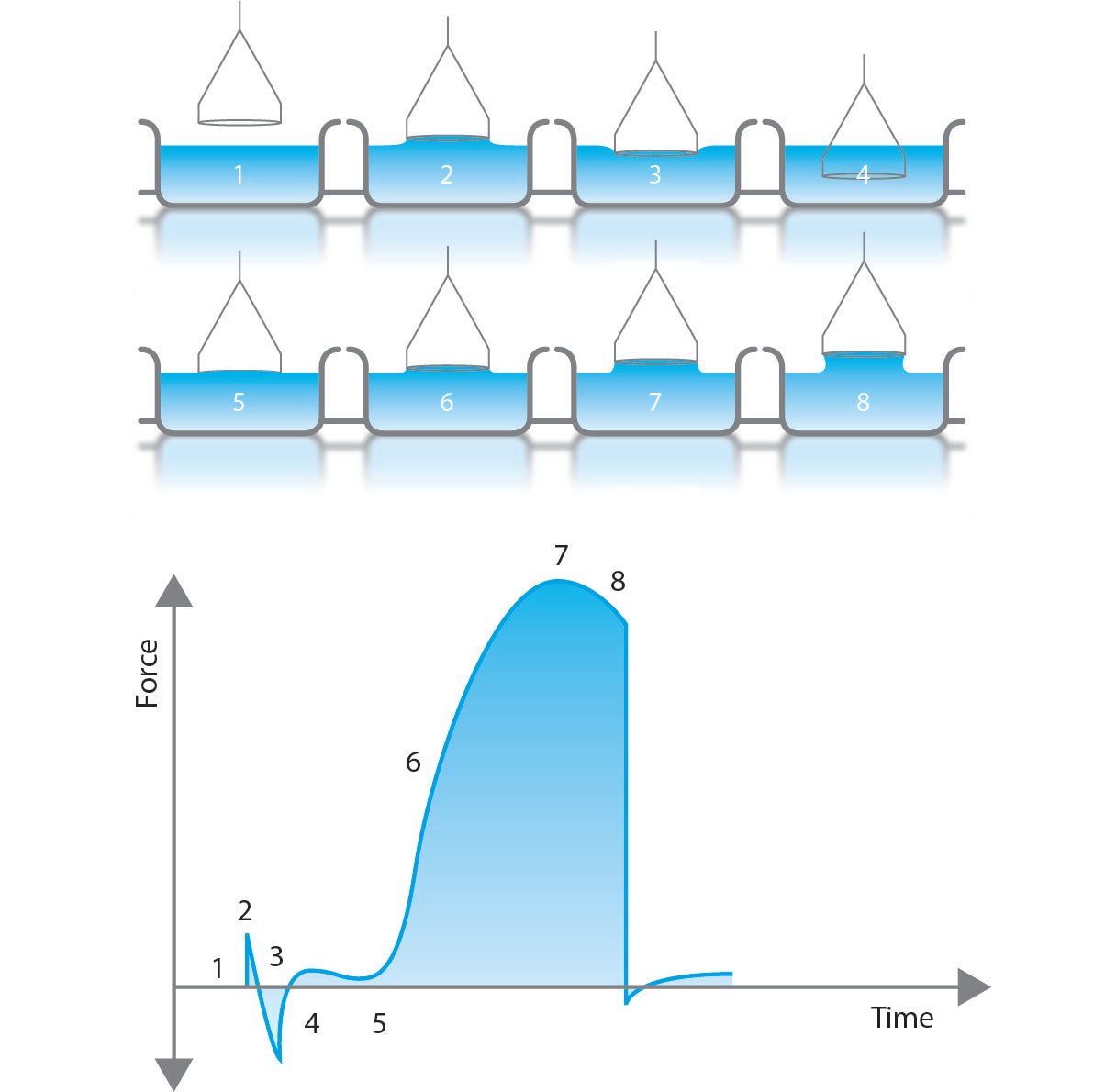
How to choose surface tensiometer or tensiometry to measure surface tension or interface tension Solution 1.18. The difference lie in the fact that “missing”cylinder add additional force and reduce the amount of liquid that has to raise. The balance between gravity and surface tension is. (1.7.15) σ 2 π ( r i cos θ i + r o cos θ o) = ρ g h ( π ( r o) 2 − π ( r i) 2) Which can be simplified as.
Source Image: biolinscientific.com
Download Image
Surface Tension Measures Which Of The Following
Solution 1.18. The difference lie in the fact that “missing”cylinder add additional force and reduce the amount of liquid that has to raise. The balance between gravity and surface tension is. (1.7.15) σ 2 π ( r i cos θ i + r o cos θ o) = ρ g h ( π ( r o) 2 − π ( r i) 2) Which can be simplified as. Sep 21, 2022The surface tension of a liquid is a measure of the elastic force within the liquid’s surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension allows objects that are denser than water, such as the paper clip shown in B in the figure below, to nonetheless
What is surface tension?
Jan 30, 2023Surface Tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface Surface Tension Measurements

Source Image: yumpu.com
Download Image
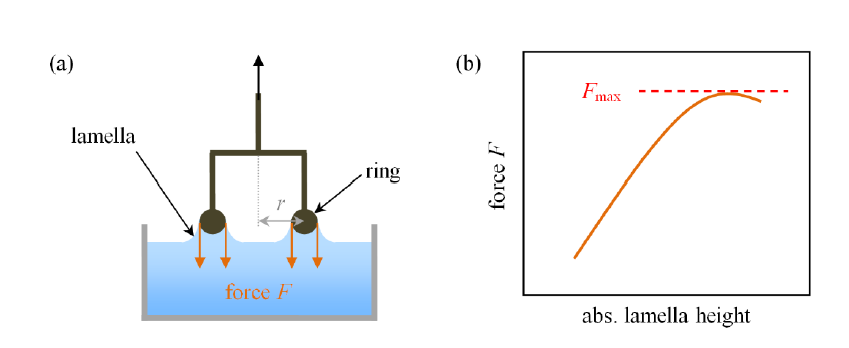
Surface tension measurement by Du Noüy ring method Jan 30, 2023Surface Tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface
Source Image: biolinscientific.com
Download Image
Four guiding principles for choosing frameworks and indicators to assess research impact | Impact of Social Sciences The viscosity of a liquid is a measure of its resistance to flow. Water, gasoline, and other liquids that flow freely have a low viscosity. Honey, syrup, motor oil, and other liquids that do not flow freely, like those shown in Figure 5.3.1 5.3. 1, have higher viscosities. We can measure viscosity by measuring the rate at which a metal ball

Source Image: blogs.lse.ac.uk
Download Image
How to choose surface tensiometer or tensiometry to measure surface tension or interface tension This page titled 6.7: Cohesion and Adhesion in Liquids – Surface Tension and Capillary Action is shared under a CC BY license and was authored, remixed, and/or curated by OpenStax. Attractive forces between molecules of the same type are called cohesive forces. Attractive forces between molecules of different types are called adhesive forces.
Source Image: surface-tension.org
Download Image
Liquid Testing with Your Smartphone | October 2021 | Communications of the ACM Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). If the surface is between two liquids (such as water and oil), it is called “interface tension.”

Source Image: m-cacm.acm.org
Download Image
Fundamentals of nursing practice exam | PDF Solution 1.18. The difference lie in the fact that “missing”cylinder add additional force and reduce the amount of liquid that has to raise. The balance between gravity and surface tension is. (1.7.15) σ 2 π ( r i cos θ i + r o cos θ o) = ρ g h ( π ( r o) 2 − π ( r i) 2) Which can be simplified as.

Source Image: slideshare.net
Download Image
8,567 Surface Tension Images, Stock Photos, 3D objects, & Vectors | Shutterstock Sep 21, 2022The surface tension of a liquid is a measure of the elastic force within the liquid’s surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension allows objects that are denser than water, such as the paper clip shown in B in the figure below, to nonetheless

Source Image: shutterstock.com
Download Image
Surface tension measurement by Du Noüy ring method
8,567 Surface Tension Images, Stock Photos, 3D objects, & Vectors | Shutterstock 5 days agoSurface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it.The molecules in a drop of water, for example, attract each other weakly. Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules.
How to choose surface tensiometer or tensiometry to measure surface tension or interface tension Fundamentals of nursing practice exam | PDF Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). If the surface is between two liquids (such as water and oil), it is called “interface tension.”